-
Preview – Deep Dive – Recent Patents & Innovations in Ni-based Cathode Materials
15 pages, addendum with patent summaries: 68 pages, version: 2024-12-26
-
Introduction
Recent innovations in nickel-based cathode materials for lithium-ion batteries reflect a product development community wrestling with multiple competing priorities: higher energy density, improved longevity, reduced raw material and process costs, and enhanced sustainability. This review analyzes patent filings and public disclosures from 2023 onwards to identify emerging patterns in how leading battery manufacturers, materials companies, and startups are addressing these challenges, in several cases in collaboration with academic research groups.
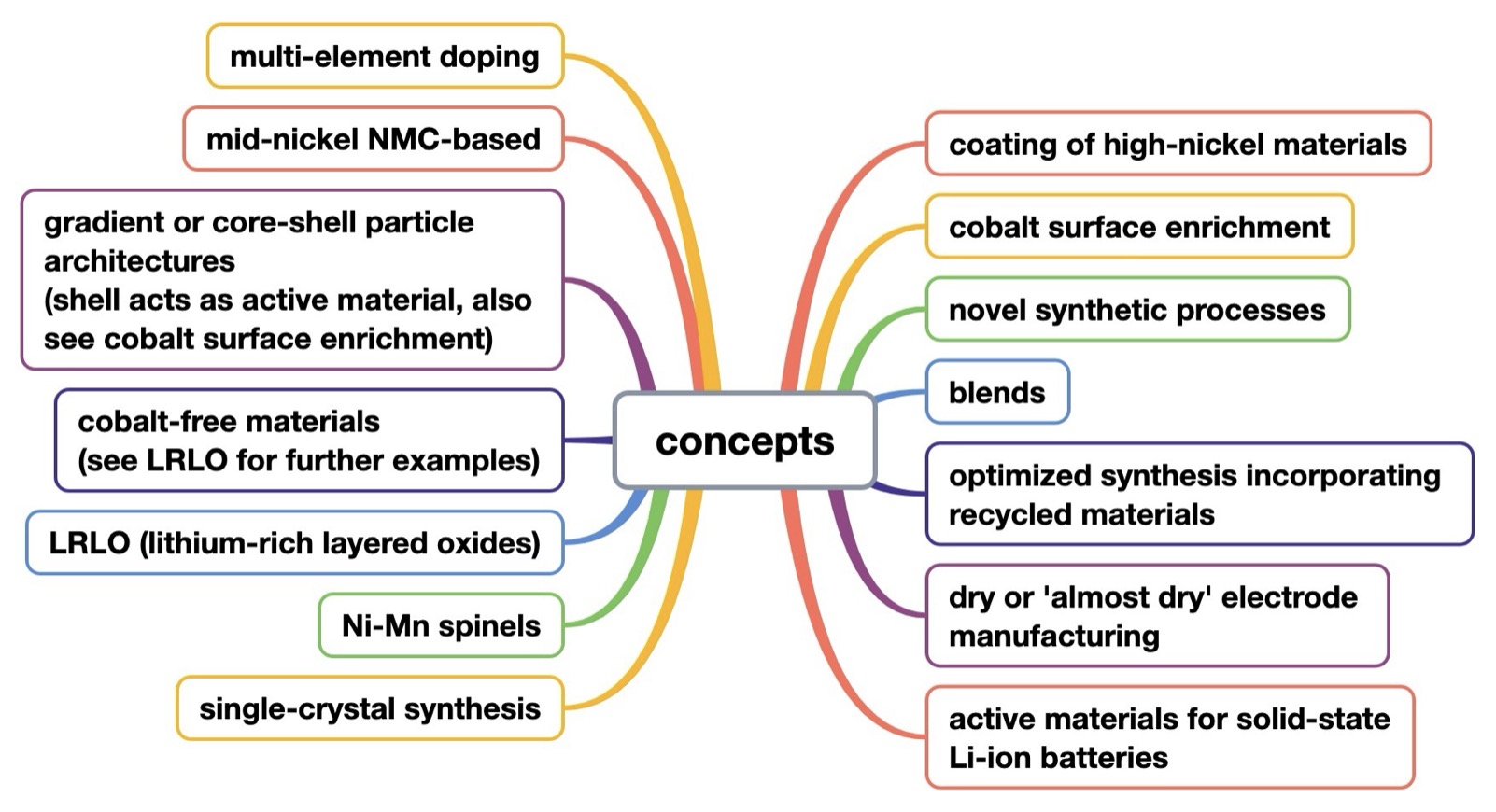
The analysis reveals 14 key concepts shaping the evolution of cathode materials (Figure A-1).
Figure A-1: technology decision tree – 14 commercially relevant concepts related to Ni-based active materials for positive Li-ion battery electrodes, identified in patent families published since 2023 (publication date of first patent family member, 2 additional earlier patent families and 2 commercialization efforts identified in public reports other than patents are included in Figures D-2 to D-15 that cover each of the 14 concepts)
|