-
Lithium-ion batteries – electrolytes – solid & semi-solid
-
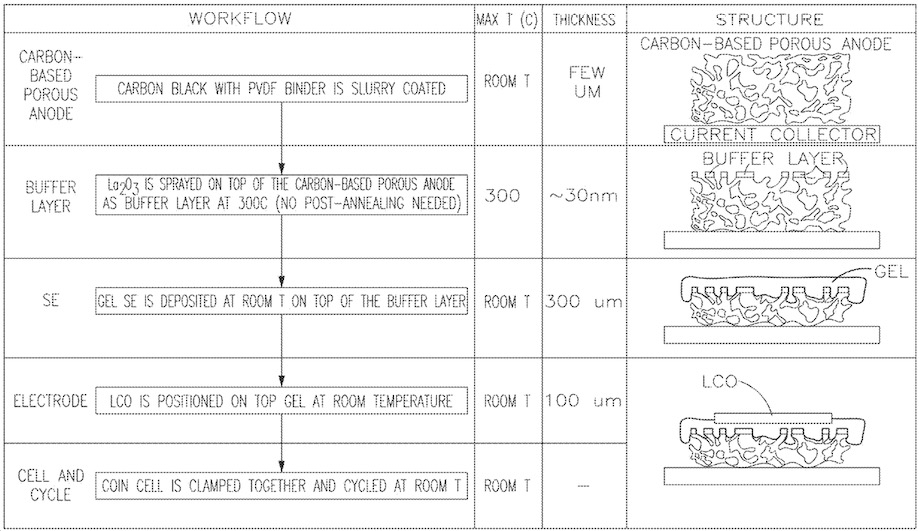
To form a negative electrode that allows for cycling of lithium metal, a carbon black / PVDF (polyvinylidene fluoride)
slurry was applied to a Cu foil, which resulted in a 5 μm thick porous MIEC (mixed ionic-electronic conductor) layer (see Figures below).
This negative electrode was heated to 300°C, and lanthanum nitrate (La(NO3)3) was spray-pyrolyzed onto it,
forming a 30 nm thick La2O3 ELI (electrolyte-layer integration) buffer layer
without post annealing.
A gel solid electrolyte was made using a PVDF-HFP (poly(vinylidene fluoride-co-hexafluoropropylene))
matrix with lithium bis(pentafluoroethanesulfonyl)imide in a 1:1 mass ratio mix of ethylene carbonate
and propylene carbonate. This electrolyte, about 300 μm thick, was layered onto the buffer layer at ambient
temperature. A 100 μm thick LiCoO2 (LCO) layer formed the positive electrode, completing the coin cell assembly.
Impedance measurements across 50 cycles (0.5 C charge / discharge) illustrate no significant increase, as compared to an increase of several orders of magnitude for
a comparative example without La2O3 layer.
FEW UM = few micrometers (about 5 μm as described above)
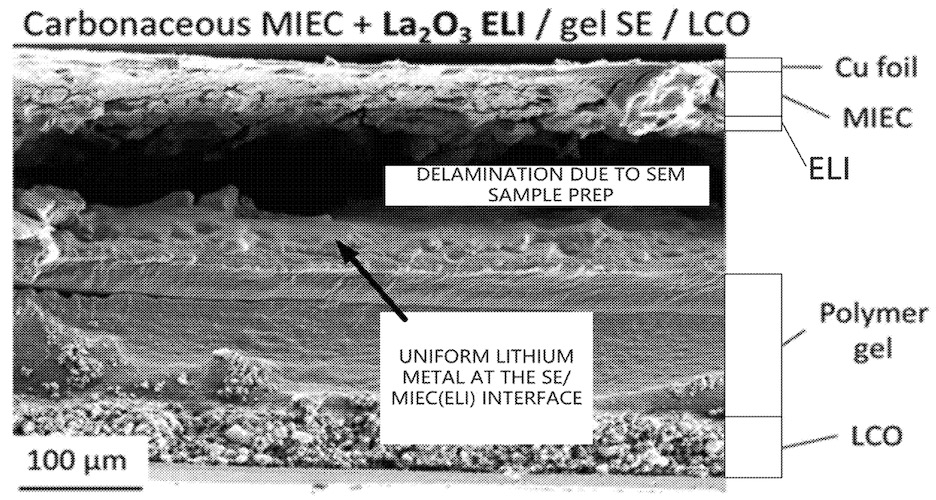
This work illustrates how the combination of a porous carbon black scaffold on copper covered by a La2O3 layer allows for cycling of lithium
metal negative electrodes with promising cycling stability (a larger number of larger-scale experiments is a necessary next validation step).
The gel solid electrolyte can probably be replaced with a liquid carbonate-free semi-solid or solid electrolyte layer to achieve an improved inherent safety
profile.
From a process cost perspective, this work is very significant because high-temperature sintering above 300°C has been avoided. The need for producing
and handling a free-standing oxide film was avoided by employing a spray-pyrolization process.
It is a very interesting question if La-free oxides based on highly abundant elements exhibit similarly favorable
characteristics as La2O3.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Get a quote to make better informed decisions.
-
Lithium-ion batteries – positive electrode
-
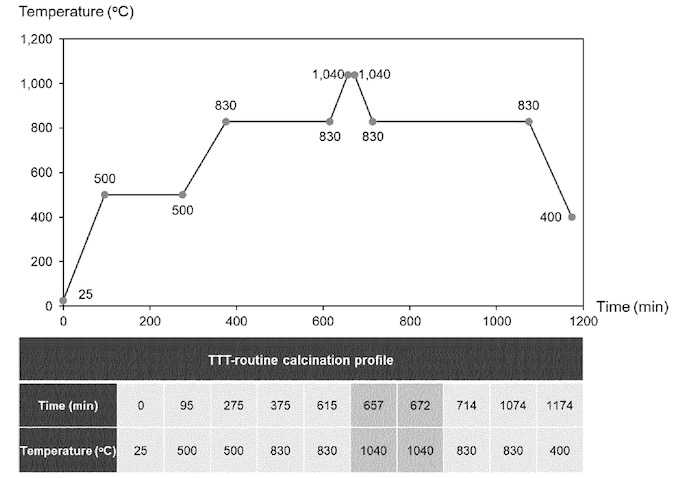
LiNi0.90Co0.05Mn0.05O2 (NMC9½½) was synthesized from the corresponding hydroxide precursor
and Li2CO3 using the temperature protocol shown in the top Figure that involves process steps at 500, 830 and 1,040°C.
The middle Figure exhibits a SEM image of the corresponding NMC9½½ that illustrates well-defined crystallites, as compared to a comparative material
(bottom Figure) prepared without the so-called transient thermal treatment (TTT) step at 1,040°C for 15 min (formation of rounded, smaller domains).
While no electrochemical data is shown, the following advantages of single-crystal as compared to polycrystalline NMC materials are emphasized in the patent:
reduced structural deterioration, electrolyte side reactions and gas generation.
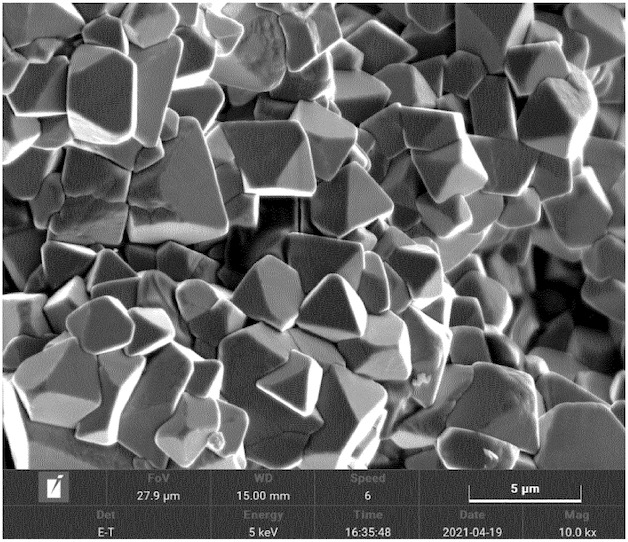
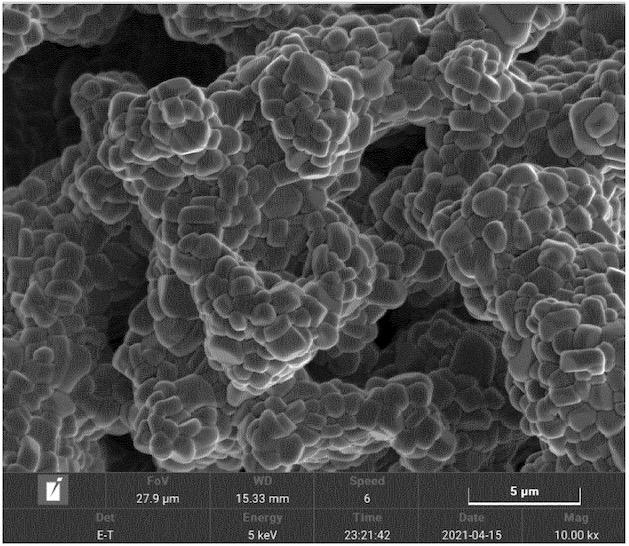
This work illustrates how a comparably short (15 min) high-temperature calcination step at 1,040°C very substantially affects NMC9½½ crystallinity
and presumably allows for benefiting from the advantages of single-crystal NMC without significantly increasing process costs.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Get a quote to make better informed decisions.
-
Lithium-ion batteries – negative electrode (excluding Li metal electrodes)
-
Silicon powder with an average particle size of about 20 µm was dispersed in 67% nitric acid for about 1 h, filtered and rinsed with water to remove any residual acid.
This treatment results in the formation of a surface oxide layer (<100 nm thickness).
Negative electrodes were built by dispersing treated silicon in a slurry with NMP (N-Methyl-2-pyrrolidone)
and polyamic acid resin, followed by coating on PET (polyethylene terephthalate) film, densification with a calender,
removal of the free-standing electrode from the PET foil, cutting, vacuum drying (120°C for 15 h, 220°C for 5 h),
and a thermal treatment (1,175°C) to carbonize the polymer.
The Si-carbon electrode was laminated with polyamide-imide-coated Cu foil to form electrodes for which the electrochemical test results
shown below were obtained (comparison of electrodes based on acid-treated and untreated Si, 0.5 C charge, 4 C discharge).
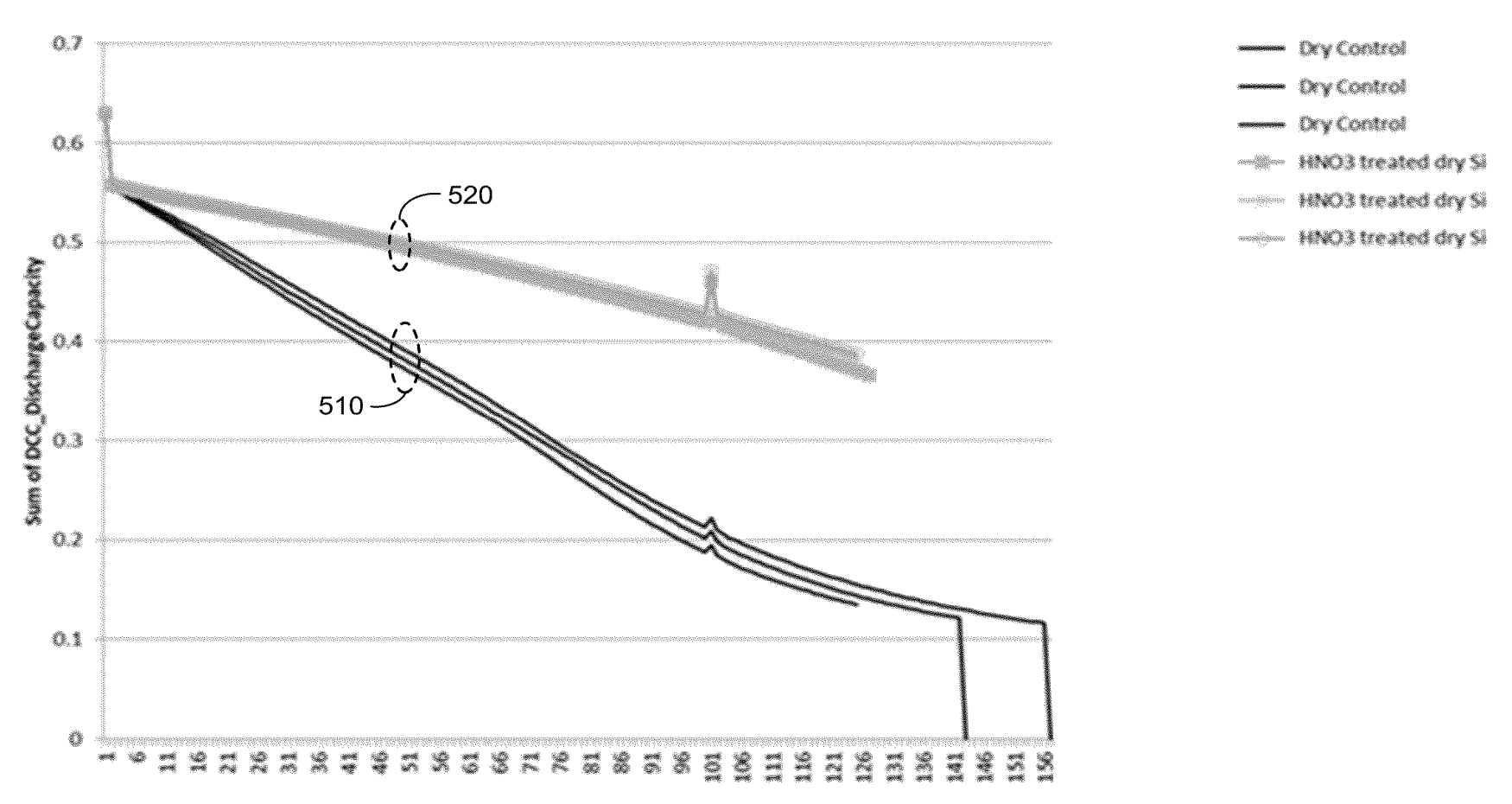
This work illustrates how the use of acid-treated Si in the context of Enevate's carbonized Si-carbon electrodes is highly beneficial
for cycling stability.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Get a quote to make better informed decisions.
-
Fuel cells (PEMFC / SOFC / PAFC / AEMFC) – electrochemically active materials
-
A platinum-free manganese cathode catalyst complex was made by mixing carbon nanotubes (CNT) and manganese (III) phthalocyanine chloride
(1:2 mass ratio).
The mixture was combined with aniline monomer (2:1 mass ratio) in water and dried at 60°C for 8 h.
The resulting powder was heat-treated at 800°C for 3 h under a nitrogen atmosphere, and dispersed in a solution of Nafion ionomer,
water, and n-propanol.
A graphene dispersion and the catalyst dispersion were injected into separate nozzles. The graphene dispersion was first sprayed onto a FEP (fluorinated ethylene propylene) material, followed by the catalyst dispersion
(ultrasonic spraying, 180 kHz, 10 μm droplets). This process was repeated to alternately layer graphene and catalyst, forming a 100-layer electrode.
Finally, a membrane-electrode assembly (MEA) was created by using this layered structure as a cathode (4 mg/cm2 cathode catalyst loading)
and a Pt/C electrocatalyst as an anode,
hot-pressed onto a polymer electrolyte membrane. Tests were also made with a comparative MEA with 0.2 mg/cm2 Pt-based cathode
catalyst.
A similar initial current density was obtained between the two MEA's. The Mn-based MEA exhibits a current density retention of 91% after 100 h at 0.7 V
as compared to 33% for the Pt-based comparative MEA. Favorable results were also obtained upon use of Fe or Co instead of Mn.
This work illustrates how the definition of a very specific 100-layer graphene-manganese structure enables promising longevity
in cathode PEMFC catalysts.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Get a quote to make better informed decisions.
-
Triweekly patent lists for other categories (Excel files are included for premium users)
-
- Lithium metal containing batteries (excluding Li-S, Li-Air): XLSX
-
- Lithium-ion batteries – electrolytes – liquid: XLSX
-
- Lithium-ion batteries – separators: XLSX
-
- Lithium-sulfur batteries: XLSX
-
- Metal-air batteries: XLSX
-
- Na-ion batteries: XLSX
-
Prior patent updates
-
2023-10-31
-
2023-10-10
-
2023-09-19
|