-
Lithium-ion batteries – electrolytes – solid & semi-solid
-
A coated active material was developed comprising a positive electrode active
material and a dual-layer coating system (see Figure below).
The first solid electrolyte with composition
Li2.7Ti0.3Al0.7F6 was prepared through
ball milling of LiF, TiF4, and AlF3 precursors (molar ratio
2.7 : 0.3 : 0.7) using a planetary ball mill (500 rpm, 12 h). The second solid
electrolyte with composition
Li2.5Zr0.5Y0.5Cl6 was
synthesized from LiCl, ZrCl4, and YCl3 precursors (molar
ratio 2.5 : 0.5 : 0.5) under similar conditions.
The first layer was formed by compression-shear treatment (6,000 rpm, 50 min) with
a mass ratio of NMC to first solid electrolyte of 100 : 3. The second layer was
subsequently applied with a mass ratio of 100 : 0.5 using the same treatment
conditions.
Button cells were assembled using the coated active material in positive
electrodes with mass ratios of coated active material : Li2.5Zr0.5Y0.5Cl6 :
carbon nanofibers (Resonac) = 64 : 34 : 2. The cells demonstrate improved performance
with initial discharge capacity increases of 5.5% compared to single-layer
coated materials. Pulse discharge voltage at -40°C exhibits significant improvements,
reaching 2.057 V (0.5 s) and 1.979 V (1 s) for the optimized dual-layer system
compared to 1.942 V and 1.897 V respectively for the comparative single-layer
system.
The dual-layer coating structure enables reduced interfacial resistance between
the active material and solid electrolyte while maintaining excellent ionic
conductivity. The first layer provides effective protection against electrolyte
decomposition, while the second layer facilitates improved ionic transport at the
electrode-electrolyte interface.
100: coated active material
110: positive electrode active material
111: first layer
112: second layer
120: coating layer
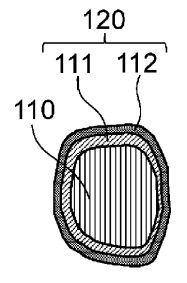
Unexpectedly, the outer layer of the dual-layer coated NMC active material is identical to the bulk catholyte material
present in the positive electrode (Li2.5Zr0.5Y0.5Cl6).
Possibly, this approach allows for protection and maintenance of uniformity of the inner
Li2.7Ti0.3Al0.7F6
layer. This makes a lot of sense because while the inner layer plausibly exhibits favorable protection against electrolyte decomposition,
it's ionic conductivity is substantially lower than that of the outer layer Li2.5Zr0.5Y0.5Cl6.
If the outer coating layer of an active material is identical to the catholyte, the chance of achieving low interface resistance
is naturally favorable in conjunction with favorable mechanical deformability characteristics for which halides are generally known.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Lithium-ion batteries – negative electrode (excluding Li metal electrodes)
-
Silicon-based composite material particles were coated with an artificial SEI
(solid electrolyte interface) film containing boric acid compounds.
Porous carbon was prepared from sucrose by carbonization (800°C, 2 h,
N2 atmosphere) followed by KOH activation (mass ratio 1 : 2, 800°C,
2 h).
The silicon-carbon composite material was prepared by CVD (chemical vapor
deposition) using monosilane gas. The porous carbon was heated (600°C,
N2 atmosphere), then exposed to SiH4 / N2
gas mixture (20% SiH4 by volume, 600°C, 30 h), resulting in
nano-silicon particles dispersed within the carbon matrix pores and surface.
2-Fluorophenylboric acid was dissolved in ethanol to form a 1 mass% solution. The silicon-carbon composite
material was added to the boric acid
solution, mixed uniformly, then dried (70°C) and heat-treated
(150°C, 4 h, N2 atmosphere, 3°C/min heating rate). The resulting
material is coated with 2-fluorophenylboric acid (2 mass%, 2-10 nm).
The coated
silicon-based composite material exhibits a BET specific surface area of
1.53 m2/g.
In half-cells, the material exhibits a reversible capacity of
1,830 mAh/g, a first cycle efficiency of 93.1%, and a capacity
retention of 98.7% after 100 cycles (1 C charge / discharge), as
compared to 1,825 mAh/g, 92.1%, and 94.8% for an uncoated comparative silicon-carbon
material, respectively.
Full-cell tests with NCM811-based positive electrodes exhibit improved
high-temperature storage performance, with a capacity retention of 87.4%
(60°C, 14 days), compared to 81.7%
for the uncoated material. The boric acid compounds containing fluorine
elements enhanced SEI film stability and ionic conductivity.
This work suggests that 2-fluorophenylboric acid is well-suited as coating material for Si-carbon composite materials,
resulting in improved electrochemical characteristics. Cost-effective up-scaling of the coating process should
be feasible.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Lithium-ion batteries – positive electrode
-
A lithium manganese-based oxide positive electrode active material was synthesized
through co-precipitation of NiSO4·6H2O and
MnSO4·H2O (40 : 60 molar ratio) using NaOH and
NH4OH as precipitation agents (pH 9.0, 60°C, 24 h). The synthesis was
conducted under N2/air atmosphere (9 : 1 volume ratio) to obtain
Ni0.4Mn0.6(OH)2 precursor particles
(D50: 12.0 μm).
The precursor underwent a two-step heat treatment: first at 550°C for 5 h in air,
followed by mixing with LiOH (Li/other metals molar ratio = 1.25) and heated at
900°C for 8 h under O2 atmosphere. The resulting
oxide exhibits C2/m and R-3m space groups as a
solid solution.
SEM analysis reveals that secondary particles consist of primary particles
with oriented structure in the shell region (no Figure identified in patent). The primary particles align with
their long axes oriented from center to surface, exhibiting elongated morphologies
with average dimensions of 250 nm (long axis) and 105 nm (short axis).
The material exhibits a BET specific surface area of 1.7 m2/g. In half-cells, the material exhibits a discharge capacity
of 230.5 mAh/g, a first charge coulombic
efficiency of 88.8% at a
0.1 C rate (2.0-4.6 V vs. Li+/Li). Rate capability testing exhibits 81.0% capacity
retention at 2.0 C compared to 0.1 C.
A comparative material without oriented structure exhibits significantly worse
performance (116.7 mAh/g discharge capacity, 15.4% rate capability), confirming
that the oriented primary particle structure enhances Li-ion transport kinetics.
This work illustrates an 'overlithiated' Ni-Mn active material with favorable primary particle alignment (orientation from
center to surface) that exhibits promising electrochemical performance.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Fuel cells (PEMFC / SOFC / PAFC / AEMFC) – electrochemically active materials
-
A fluorine-free polyacrylate with C4-C35 alkyl side chains
was applied to carbon fiber gas diffusion layers (GDL) for polymer electrolyte fuel
cells via in-situ crosslinking (room temperature to 100°C).
The polyacrylate polymer exhibits functional groups X (hydroxy, carboxy, amino
groups) that crosslink with functional groups Y (isocyanate, carboxy, amino
groups) through a bi-functional isocyanate crosslinker. Additional functional groups Z (isocyanate groups) form covalent bonds
between the hydrophobic coating and carbon fibers of the GDL.
Carbon fibers were pretreated via plasma treatment to enhance surface reactivity.
The polymer was applied as an aqueous dispersion containing an organic co-solvent
and surfactant via a padding technique, followed by drying (80°C).
The resulting hydrophobic coating exhibits a water contact angle of ≈120°
with minimal contact angle hysteresis between advancing and receding angles,
enabling effective water droplet roll-off from the GDL surface.
The process eliminates high-temperature PTFE sintering (>300°C), enabling the
simultaneous application of microporous layer and catalyst layer in a single
process step, potentially improving electrical conductivity and mass transport.
No electrochemical performance data was disclosed.
This work suggests that favorable GDL properties can be obtained without the use of fluorinated polyvinylidene
difluoride (PVDF) / polytetrafluoroethylene (PTFE) polymers.
-
The premium version includes another two patent discussions, plus an Excel list with 50-100 commercially relevant recent patent families.
-
Triweekly patent lists for other categories (Excel files are included for premium users)
-
- Lithium metal batteries (excluding Li-S, Li-Air): XLSX
-
- Lithium-ion batteries – electrolytes – liquid: XLSX
-
- Lithium-ion batteries – separators: XLSX
-
- Lithium-sulfur batteries: XLSX
-
- Na-ion batteries: XLSX
-
Prior patent updates
-
2025-06-10
-
2025-05-20
-
2025-04-29
-
2025-04-08
-
2025-03-18
|