ALL-SOLID-STATE BATTERY COMPRISING SILICON-CONTAINING LAYER
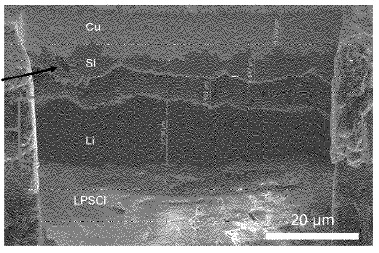
An anode-free all-solid-state battery was developed featuring an amorphous silicon layer on a copper current collector. The silicon layer exhibits a thickness of up to 500 nm (see Figure), resulting in Ns/P ratios (charge capacity of silicon layer to positive electrode) of less than 0.3.
The battery comprises a Li6PS5Cl (LPSCl) solid electrolyte separator and a positive electrode composite of NMC811 with LPSCl and vapor-grown carbon fiber (VGCF) (mass ratio : 66 : 31 : 3, 3 mAh/cm2 loading). Amorphous silicon (500 nm) was deposited on Cu via sputtering. This stack was pressed at 370 MPa prior to cycling, while a stack pressure of 5 MPa was maintained during cycling.
During initial charging, silicon lithiation occurred up to 3.7 V (0.25 mAh/cm2), forming amorphous LixSi1-x (0.5<x<0.79) with composition Li0.7Si0.3. Subsequent lithium metal plating (2.75 mAh/cm2) formed a dense lithium layer on the pre-lithiated silicon substrate. The lithiated silicon layer persists throughout cycling without further silicon participation.
Rate performance testing in the all-solid sulfide electrolyte cells exhibits stable cycling up to C/5 (0.6 mA/cm2). Thin-film cells with LiPON solid electrolyte demonstrate critical current densities up to 5.0 mA/cm2, representing a five-fold improvement compared to bare Cu current collectors.